Independent assessment plays a crucial role in NASA’s long-term success by addressing essential questions requiring rapid response to support further program development. The Office of the Chief Health and Medical Officer (OCHMO) uses this process as a tool for decision-making and providing unbiased insight into issues that arise concerning crew health and safety. Technical Interchange Meetings (TIMs) allow NASA subject matter experts (SMEs) to consult with outside experts from industry, academia, hospitals and clinical settings, and the military to better understand specific risks, gain knowledge of current best practices and cutting-edge research occurring outside NASA, and to answer questions on topics the NASA SMEs develop and share prior to the meeting. Results of these independent assessment TIMs are incorporated into the NASA-STD-3001 documents, future editions of OCHMO technical briefs, informative summary reports, and/or program technical memos.
Learn More About Previous OCHMO Independent Assessments
Assessment of Oxygen Toxicity and Neurovestibular Disturbances during Neutral Buoyancy Laboratory (NBL) Exploration Spacesuit Testing
In March 2025, NASA’s Office of the Chief Health and Medical Officer (OCHMO) initiated a working group to investigate operations in the Neutral Buoyancy Laboratory (NBL) and determine the possible causes of adverse physiological effects that have been experienced by subjects operating exploration spacesuits during 1/6G simulations in the NBL training platform.
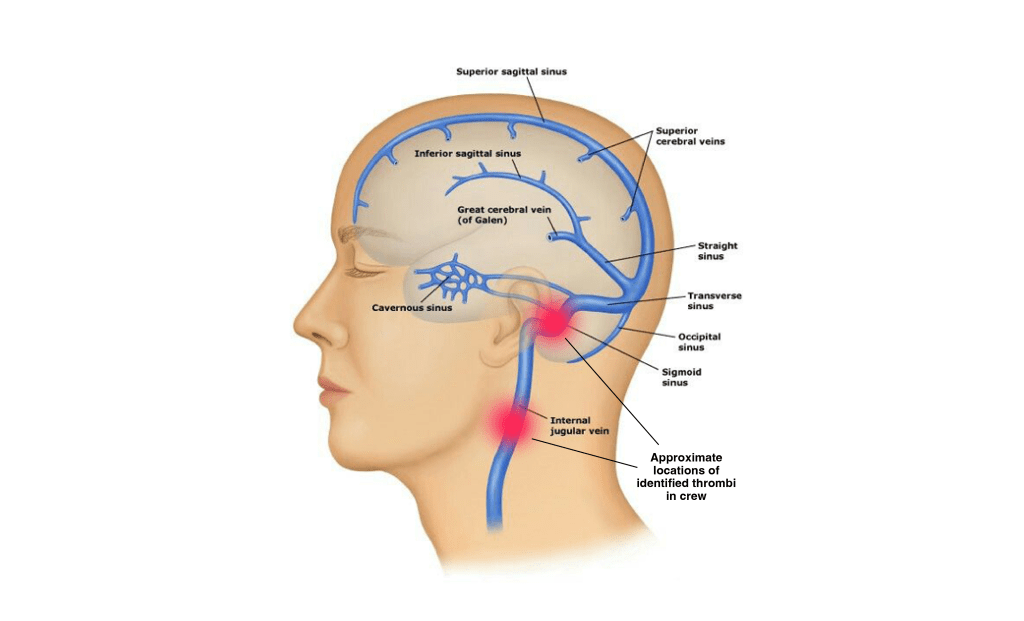
Venous Thromboembolism During Spaceflight
In October 2024, NASA’s Office of the Chief Health and Medical Officer (OCHMO) initiated a working group to review the status and progress of research and clinical activities intended to mitigate the risk of venous thromboembolism (VTE) during spaceflight. The working group took place over two days at NASA’s Johnson Space Center a second meeting on the topic was held in December 2024 at the European Space Agency (ESA) facility in Cologne, Germany.
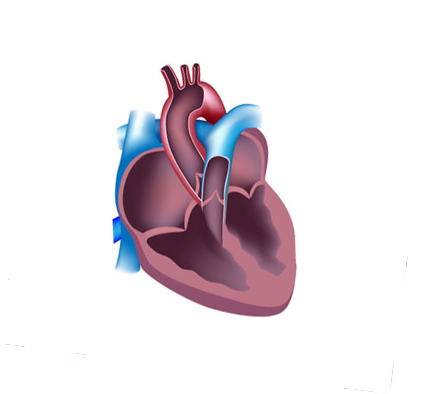
Patent Foramen Ovale (PFO)
NASA’s Office of the Chief Health and Medical Officer (OCHMO) initiated a working group to review and provide analysis on the status and progress of research and clinical activities intended to mitigate the risk of decompression sickness (DCS) issues as related to Patent Foramen Ovale (PFO) during spaceflight and during associated ground testing and human subject studies involving decompression. The working group took place over two days at NASA’s Johnson Space Center (June 4 & 5, 2024).

Hydrogen Sulfide (H2S)
NASA’s Office of the Chief Health and Medical Officer (OCHMO) assembled a small working group to review hydrogen sulfide (H2S) Spacecraft Maximum Allowable Concentration (SMAC) values. The group met virtually three times during February and March 2023, and panel members submitted individual opinion statements in April 2023.
13th IAASS Conference 2024 on Human System Standards
This paper describes NASA’s Agency-level Space Flight Human System Standards and technical briefs that prescribe technical requirements for crew selection, medical operations, and vehicle design that enables human space flight missions by minimizing health risks to astronauts, providing vehicle design parameters that maintain astronaut safety and enable the performance of both flight and ground crews. NASA human space flight standards provide knowledge, guidelines, thresholds, and limits for crew medical selection, the successful design and operation of human rated spacecrafts, and missions. These standards cover many topics including medical and behavioral care in space, limits to protect health outcomes, launch and landing accelerations limits, extravehicular suit design, radiation exposure, monitoring, and shielding, environmental parameters (oxygen concentration, carbon dioxide levels), food and nutrition requirements, microbial control, net habitable volume, acoustic limits, maximum allowable concentrations of compounds, autonomy, task analysis, maintainability, and usability of vehicle systems.
Learn More
Human Research Program Investigation Workshop (HRP IWS)
Presentations and Publications

2025 Abstract – NASA OCHMO Approach to Crew Survivability: An Analysis for Future Spaceflight Missions

2025 Presentation – NASA OCHMO Approach to Crew Survivability: An Analysis for Future Spaceflight Missions

2025 Abstract – NASA Spaceflight Medical Selection, Recertification and Mission Evaluation Standards: Forward Work for Future Long-Duration Exploration Missions

2025 Presentation – NASA Spaceflight Medical Selection, Recertification and Mission Evaluation Standards: Forward Work for Future Long-Duration Exploration Missions

2025 Poster – Incorporation of Human Risk Directed Acyclic Graphs (DAGs) With Mishap Investigations to Un-Silo Knowledge
2024 Abstract – NASA Spaceflight Human-System Standard Maintenance: An Evolving Strategy to Keep NASA Agency-Level Standards Current Through Partnerships with the Scientific Community

2024 Presentation – NASA Spaceflight Human-System Standard Maintenance: An Evolving Strategy to Keep NASA Agency-Level Standards Current Through Partnerships with the Scientific Community
npj Microgravity Publication
The purpose of this paper is to describe NASA’s approach to establishing and maintaining a set of Agency-level Space Flight Human System Standards managed by the Office of the Chief Health and Medical Officer (OCHMO) at NASA that enables space flight missions by minimizing health risks to astronauts, providing vehicle design parameters, and supporting the performance of both flight and ground crews.
Read It Here about npj Microgravity Publication
Contact Us
Need further help with Human Health and Spaceflight Standards?
We will never share your email address.