Transcript
(music)
[0:06] Narrator: You could say that NASA came out of thin air.
NASA’s roots are in aeronautics, the study of how objects move through air. In 1915, the United States government formed the National Advisory Committee for Aeronautics, or NACA to develop airplane technology for World War I.
For more than 40 years, NACA aeronautic research and experiments led to ever-more advanced planes, including ones that could fly supersonically – faster than sound. As we sought ways to go ever faster and higher, and even fly beyond Earth’s atmosphere and travel to the Moon, NACA evolved into NASA, the National Aeronautics and Space Administration.
“The Right Stuff” movie trailer
Announcer: This is the story of the special few at the very top. The elite brotherhood whose achievements inspired the Nation, and captured the imagination of the world. These are the men who had The Right Stuff.NASA pilot: They all want to see Buck Rogers, and that’s us.”
[1:18] Narrator: Many NASA research and test pilots these days are more focused on Earth than on space, modifying aircraft and flying them on scientific missions around the world. Pilot Gerrit Everson is chief of flight operations at NASA’s Wallops Flight Facility in Virginia.
Gerrit Everson:The test pilot component is testing the airworthiness of our aircraft modifications to prove that they’re safe to fly a science mission, and then the research piloting really relates to working with teams of researchers to take them wherever they need to go and bring home the data. We’re also the link between the project teams and the aircraft, so we can help them understand what the capabilities of the airplane are.
You know, sometimes you get some oddball requests to fly at 50 feet. Well, we’re not going to do that. And then, “Hey, we need to go 500 miles an hour in the P-3 Orion.” Well, it’s a turboprop aircraft, so that’s not going to happen either. So we tell them what we can and what we can’t do within reason, and also help mitigate the risks of the mission if it happens to be a bit hazardous.
[2:13] Narrator: Turboprop planes like the P-3 Orion use propellers to fly through the air. In the quest to help scientists gather information about our planet’s air and weather, Gerrit has flown this plane in some unusual ways.
Gerrit Everson:We have flown a mission called IMPACTS, which is the Investigation of Microphysics and Precipitation for Atlantic Coast Threatening Snowstorms. And I only knew that because it says it in front of me; every mission has a long acronym and I can never remember what they stand for. This was with our P-3 Orion, and we would fly through winter snow storms, particularly over the Northeast.
(sound FX: propeller plane P-3 Orion and wind)
So it was your typical garden variety Nor’easter snowstorm that was of interest to the scientists, and particularly how snow and ice particles form, and how snow bands form in these winter storms. We would fly around-the-clock operations out of Wallops Flight Facility. At least we would prepare to, and it’s a storm-driven program, so you really didn’t know what your flight schedule was going to be until a day or two prior, when a storm forms. And then our teams of researchers would have a good understanding of where the flight path should be. That’s where the research pilots would interact with them and start telling them what we can, what we can’t do, and, “Hey, this is a better flight path then what you’re proposing, because it reduces flight over in New York.” You know, everyone loves to help out NASA, but you can’t plow through approach corridors right over New York City.
[3:34] We would fly right through the snow bands measuring the snow particles, and it wasn’t uncommon to hear from air traffic controller, “NASA, NASA, four-two-six, be advised, heavy to extreme precipitation on your nose for the next 20 miles.” And we’d acknowledge their call and thank them for it, but we would fly right through it. We have good anti-icing and de-icing systems and it’s a very sturdy aircraft. The P-3 is a former Navy anti-submarine aircraft that’s built like a tank. So it can really withstand quite a bit that you wouldn’t do with an airliner.
(sound FX: turbulence)
Turbulence is sometimes a factor when you’re flying through a bad storm, and in one case we had about 150-mile-an-hour wind at about 22,000 feet out of the west, which is a pretty huge crosswind at that altitude from a big winter storm. And it was pretty turbulent.
Some of the more challenging flights are ones over very congested areas. So one is a mission that was called DISCOVER AQ. Again, a big acronym, and I’m not even going to pretend to remember what exactly the acronym stands for, but in this case, the back of the plane was at capacity, with teams of researchers and the scientific instruments — it looks like a flying chemistry lab. We would fly over major metropolitan areas, and we would fly from 17,000 feet all the way down to the surface in a very tight spiral to measure air quality. So we do that all day long: climb to 17,000 feet, spiral all the way down, climb back up, spiral all the way down. If you get motion sickness, it will make you sick.
[4:57] The flying wasn’t challenging, meaning the stick-and-rudder skills, but deconflicting that with all the other air traffic over Washington, DC, Baltimore, Houston, Los Angeles — that is a challenge. So in that case, we would have three pilots on board — two pilots at the controls at any given time, and another pilot that’s on the radio with air traffic control the entire time, and also on the satellite phone coordinating every maneuver that we’re going to do so that we can do it safely. You can imagine that flying in that environment, there’s always the risk of a mid-air collision, and that’s something we need to do everything we can to avoid.
Narrator: Gerrit is well-suited to navigating air space in a range of difficult conditions. Before he was a NASA pilot, Gerrit flew Navy F-14 jets on aircraft carriers, just like those seen in the movie, “Top Gun.”
(music from the movie Top Gun)
[5:42] Narrator: Aircraft carriers don’t have long runways, so to land on the boat and not end up in the water, the pilot has to fly so that a hook on the underside of the plane’s tail connects with a steel wire on the ship. When the hook meets this “arresting wire,” a hydraulic system on either side of the wire absorbs the plane’s energy, and brings it to a stop.
Gerrit Everson:I enjoyed flying around the aircraft carrier. It’s a very dynamic environment, and the Navy trains you very well, and they have a darn good safety record for something that’s extremely hazardous. And it’s tough, particularly landing on the aircraft carrier at night, because it’s a very unnatural thing to do — particularly in a big airplane, like the F-14. The tail hook that catches the wire is about 20 feet below the pilot’s eyes. And there’s 62 feet of airplane behind you. So when you’re coming to land, there’s not a whole lot of flight deck left when that thing finally touches down and catches the wire. So if you fly the airplane to put it where you think you should be, you’re way too low.
So you have to learn to just fly the instruments and look at nothing else. And it’s never a dull moment. The conditions are never the same. You could have strange winds, a pitching flight deck, bad weather, all those variables factor in to make it a challenge each and every time.
[6:56] And psychologically it can be tough, because the stakes are high when there’s a thousand people watching you, and oftentimes you don’t have a tremendous amount of fuel to keep going around. And you also have to think that the aircraft carrier just doesn’t have the sea space to just keep running into the wind to try and catch all the airplanes. So you really need to be on your game each and every time. Learning to keep calm under all those circumstances is tough to do.
(sound FX: prop plane)
I definitely apply all that for all of our NASA operations, making sure that everything that we do is safe and we’re not putting anyone in harm’s way. You know, some of the flying that we do here is more benign, where it’s not quite as difficult. Something like losing an engine in flight, that might alarm some people, but we’ve all had that happen. It’s not the worst thing in the world.
It happened outside of Barbados one year crossing the Atlantic. We were flying to Ascension Island, which was a remote volcanic Island in the middle of the South Atlantic. And shortly after takeoff, we had to do an emergency shutdown of one of the engines on the P-3. And we have four of them, so there’s plenty of redundancy. But it’s a much more hazardous situation, so we had to come back to do an emergency landing. So that alarmed some of our scientists, but all the pilots kept cool. It wasn’t a huge deal.
[8:05] Narrator: Gerrit enjoys being part of scientific missions, learning about our planet as he flies through different layers of Earth’s atmosphere.
Gerrit Everson:I find it rewarding because you see the fruits of your labor. You’re not hauling the mail or moving passengers from point A to point B; you’re actually doing cool scientific research that really makes a difference for NASA and our country. So this is much bigger than yourself, or just flying a cool airplane. It’s achieving a very important mission that you really don’t get to do anywhere else. And you get to do some incredible flying and go to some incredible places that no one else gets to see.
You also have an opportunity to listen in on the scientists, and ask questions on what they’re seeing, what the data is, how the data will be used. So you have that opportunity to ask as many questions as you’d like to, and some of these eight to 10 hour missions, there’s a long time in the plane. So you might as well learn something.
(intro music)
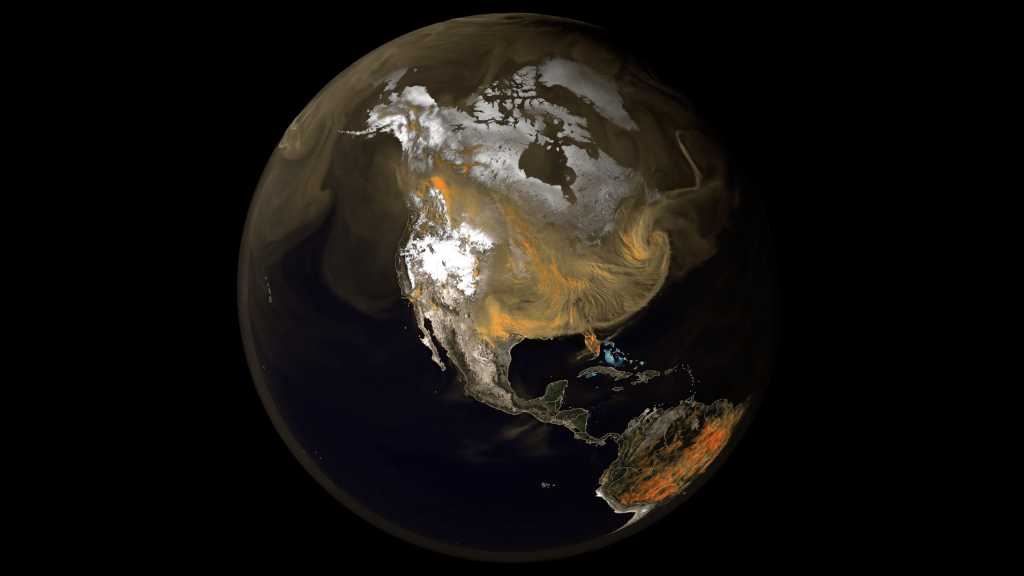
[9:28] Narrator:Welcome to “On a Mission,” a podcast of NASA’s Jet Propulsion Laboratory. I’m Leslie Mullen. JPL is in Los Angeles County, where thick smoke from wildfires recently made the air hazardous to breathe. The smoke drifted west across the Pacific Ocean and east across the US and the Atlantic Ocean, and also traveled high up in the atmosphere, creating hazy conditions wherever it went, showing us plainly that we’re all living in the same air bubble.
This is season 3, episode 6: Air and Shield.
(music)
Narrator: The phrase “I can’t breathe” may best sum up the year 2020, not only due to the record wildfires that ravaged many regions around the world, but also because of the widespread protests sparked by George Floyd, and of course, the COVID-19 respiratory virus and the muffling face masks needed to slow the spread of the global pandemic.
[10:27] NASA satellites provide overhead views on how some of these events have affected our planet. Missions like Terra and Aqua, for example, have been tracking the spread of wildfires and their plumes of suffocating smoke.
Satellites and instruments flown on planes also have been tracking how pandemic-related reductions in daily activity have altered the amount of pollution around the world. An online dashboard, created by a collaboration between NASA and the European and Japanese space agencies, details the many ways changes in human activities have affected Earth’s air, land and water. The web address for that dashboard is E-O-DASHBOARD-DOT-ORG.
For instance, the dashboard provides information on levels of nitrogen dioxide — NO2 – a pollutant released into the air when we burn fossil fuels like diesel, gasoline, and coal. Fewer vehicles on the road this year led to noticeable reductions in nitrogen dioxide, but scientists are still studying where the drops in NO2 have been the most dramatic, and tracking how long those reductions will last.
[11:41] The molecules that make up the air have changed over our planet’s history. Earth’s first atmosphere more than 4 billion years ago is thought to have been mostly hydrogen gas, with some water vapor, methane and ammonia – more like the atmospheres of Jupiter and Saturn today. Volcanoes and asteroid impacts altered our primal atmosphere, adding nitrogen and carbon dioxide to the air by about 3.4 billion years ago. After life came to be and began to use sunlight for fuel, our atmosphere made another major shift. Plants, algae, and other organisms that perform photosynthesis take carbon dioxide from the atmosphere and release oxygen as a waste product. By about 2.4 billion years ago, they were expelling significant amounts of oxygen into the air.
(music)
[12:34] The oxygen content of the atmosphere has fluctuated a lot since then. When oxygen was first introduced, it reacted with other atmospheric gases and was absorbed by the ocean. So it took a long time for large quantities of oxygen to build up in the air, and any big changes with the planet’s geology, chemistry, or oxygen-producing life would upset the balance of freely available oxygen.
Earth’s air today is about 21 percent oxygen, 78 percent nitrogen and less than one percent argon. There’s also trace amounts of other gases, like carbon dioxide. Our breathing is the opposite of plants – we take in oxygen to fuel the cells of our body, and exhale carbon dioxide. We can tolerate a little variation in the amount of oxygen in our air. According to the Occupational Safety and Health Administration, or OSHA, the best oxygen levels for humans are between 19.5 and 23.5 percent.
[13:39] But our planet’s atmosphere isn’t just important for breathing – it’s also our shield, protecting us from the extreme cold of space and the intense radiation of the Sun. Without an atmosphere, our ocean, lakes and rivers would have boiled away long ago. Our atmosphere even mutes the danger of asteroids, causing smaller ones to burn up before ever reaching the ground. The Northern Lights – the Aurora Borealis – are the glorious result of charged particles from the Sun colliding with oxygen and nitrogen in the upper atmosphere.
There’s no distinct line between Earth’s atmosphere and outer space. Instead, there’s a gradual thinning of the air as you move up. Gas molecules closest to the ground are pulled down the most by gravity, and the higher you go, the more diffuse the air molecules become. That’s why the air pressure is greater at sea level than on mountain tops.
[14:38] JPL scientist Annmarie Eldering first got swept into studying the air because of the smoggy skies of Los Angeles.
Annmarie Eldering:I always loved my math and science classes and started down the path of learning to be an engineer, and it’s only when I came out to Los Angeles and saw the beautiful smog that was here in the 1980s did I realize, “You know what? Air pollution is my calling.” It felt exciting because it was a tangible problem I could see, and the questions we were going to answer were actually going to make an impact. And then they were also just complicated enough that they stayed quite interesting, because between the chemistry and the things we didn’t understand and how hard it was to build computer models that could simulate it, there was always something that was just on the cusp of what you could do, and for me, that’s incredibly tantalizing.
[15:27] So I arrived here in ’88 for my PhD work at Caltech and studied air pollution. You pretty quickly realize you didn’t want to go out running around outside too hard in the afternoon, that it was wiser to get up and do your workout in the morning. I do remember being down on the Caltech campus and then around about October, the mountains appeared one day. And I was like, “Wow, there’s mountains right there!” Because I hadn’t seen them for the first few weeks of my time in Pasadena.
Geography is certainly a very important element for the situation we have in Los Angeles, because the mountains just trap the air in the basin and the winds are bringing it in from the ocean so you just pick up stuff as the air travels across. And as that’s happening, the Sun’s cooking it and the chemistry’s running fast and you’re making all sorts of junk.
My focus of my thesis was visibility. We were really learning the details of what was in the air that made the visibility so poor, and what changes could we possibly make that were going to improve it.
Narrator:Annmarie and her fellow students wouldn’t capture the air itself, but instead trap miniscule solid and liquid particles known as “aerosols” that stay suspended in the air.
[16:36] Annmarie Eldering:We had stations around Los Angeles and we would draw air through these filters to collect the air pollution on the filter and then bring it back to the lab. There were a few folks who did this very careful work where they then extracted the air pollution off the filter and put it through a gas chromatography system, and you can separate out and see what some of the different molecules are. And another student sampled the emissions from restaurant cooking, gas stations, some other places. You said, “I’m finding all this stuff up in the air and if I go look at the emission sources, this is what I see that they emit,” and you start to be able to understand why you found what you found in the air. We called it “fingerprint analysis.” That’s pretty much what it is, detective work to figure out, “Whose fingerprint am I finding in the air?”
[17:24] Some of the ones I think that are most surprising is things like the way the tires of your car are made matter, because a little bit of rubber gets worn out for those tires and you could actually see that as part of the stuff that we found up in the air. Then it was also a lot of organic materials, everything from the paint, paint thinners, stuff that comes out of cooking, things that come out of industry. All these little organics get up in the air and then they are part of what was forming the particles. That’s why you’ve seen over time reformulation of a lot of different things you might buy at the hardware store, why the gas dispensing, when you stick that hose in your car it’s got a little rubber gasket and that’s actually pulling the vapors back into the tanks rather than letting them escape to the atmosphere. There’s a lot of little changes that have happened to help reduce all these things that were being emitted.
Narrator: As satisfying as it was to capture the culprits behind LA’s bad air, the stake-outs could be brutal.
[18:26] Annmarie Eldering:I remember helping one of my colleagues for one of their field experiments and we thought, “Okay, we’re going to do this great experiment. We’re going study the smog and we’ll do it on like one of the smoggiest, hottest days that we have.” I found myself sitting in a gravel pit in Claremont tending to our air pollution equipment. It was hot and uncomfortable and irritating, almost ozone-y, smoggy air. I was just like, “What am I doing here?” That’s when I started to think that maybe using a more remote vantage point, like a satellite, could be advantageous, rather than sitting in the soup that you wanted to measure.
Narrator:Annmarie came to JPL and worked on the Orbiting Carbon Observatory-2 satellite, or OCO-2. She’s now the project scientist for the follow-up mission, OCO-3, an instrument on the International Space Station.
Annmarie Eldering:OCO-2 launched as a free-flying satellite in 2014, and when that was being built, they made a spare instrument just in case there was any kind of a problem. So we took that spare instrument and we developed this plan to go onto the Space Station. Going onto the Space Station was a very affordable thing to do, and it tells us some new things because it actually measures at different times of day, whereas our satellite is flying in a way that it goes over Earth the same time every day.
[19:48] So Orbiting Carbon Observatory-3 launched in May last year, and it’s been installed on the outside of the Space Station, on something called the Japanese Experimental Module. It’s almost like a little LEGO system; there’s 10 places you can plug instruments in. And so we plugged it in there, and now we’re looking down on Earth. We’re starting to get more data of carbon dioxide like our predecessor OCO-2 has done and is still doing, but we also do this neat little mapping thing — so over two minutes, we can measure over an area of about 50 miles by 50 miles, and get a little snapshot of the carbon dioxide.
Narrator: Sunlight that shines down on Earth also bounces off of the surface of our planet. OCO-2 and OCO-3 study this reflected sunlight.
[20:37] Annmarie Eldering:Every molecule in the world interacts with light in a very unique way. So if you measure the light really carefully, you can learn about how much carbon dioxide was in the atmosphere. You know, carbon dioxide and methane are really important climate gases, and there’s still things we need to learn about how they’re changing over time and why every year looks a little different. And so continuing to make these measurements is really important to try to answer these questions.
But clouds are everywhere and we want to look at the light that’s reflected off of Earth, so when there’s a cloud in the way, we can’t do our science. We actually find that upwards of 80 percent of the data has clouds in it and we can’t do what we want to do with it. But fortunately, we measure about a million times a day, so if you only get 20 percent of that, you still have a pile of data.
(music)
[21:33]While some missions need to look around the clouds that cover much of the sky, others are focused right on them.
Armin Sorooshian:My name is Armin Sorooshian. I’m a professor at the University of Arizona, and I’m serving as the principal investigator of a NASA mission called ACTIVATE, which stands for the Aerosol Cloud and meTeorology Interactions oVer the western ATlantic Experiment.
The key point of the mission is to study what are called “aerosol-cloud interactions.” The only way you can really make a cloud, or just a cloud droplet, is with an aerosol particle. And when an aerosol particle becomes a cloud droplet, that process is called activation.
So aerosols are a requirement for cloud formation, and it’s just based on simple thermodynamic principles that if one were to expect to make a pure cloud droplet made of water, the relative humidity needed is an abnormally high amount, you know, upwards of maybe 200, 300, 400 percent, which is crazy. You know, we don’t have relative humidities that high in Nature.
[22:49] Narrator:Aerosol particles are all around us. The smoke from a fire or the smog of a city can make the presence of aerosols painfully obvious, but even on clear days, you can often see a universe of dust motes and other tiny particles suspended in sunbeams.
Armin Sorooshian:There’s so many particles in the air. You know, where you and I are separately sitting, or just if we walk outside, there could be anywhere from a few tens of particles per cubic centimeter to maybe tens of thousands particles per cubic centimeter.
The particles are wide-ranging in terms of their size, and what they’re made of, and where they come from. The most abundant type of particles globally are dust and sea salt, on a mass basis. So sea salt of course comes from oceans, where waves break and they eject particles in the air. And dust comes from surfaces where wind blows, and dust can get up in the air. And then there’s biogenic forms of aerosol from plants, and also there’s pollution from fossil fuel combustion, you know, even things like meat cooking. So it’s a very diverse set of sources.
[24:01] But only a subset of those particles can become a cloud droplet. And we call those special particles CCN — stands for Cloud Condensation Nuclei. Part of ACTIVATE is to really understand that process of what we call activation. We want to know what really drives the particles that activate into cloud droplets. What’s special about them?
And then when you do make the cloud, what drives the evolution of the cloud? Because you know, clouds have a finite lifetime. And one thing in particular that intrigues me is not just how particles affect clouds, but the reverse direction. How do clouds themselves affect particles?
A lot of my graduate school work and still current work is about something called cloud processing. So we’ve already talked about how you need particles to make droplets, but then when you have a droplet in a cloud, what happens inside that droplet during the life of the cloud is actually quite interesting.
[25:06] These cloud droplets are very efficient reactors, because when that cloud droplet evaporates, the properties of that particle will be quite different than the original particle that made that droplet. And so that’s an example of how clouds can affect aerosols. And one of the enticing things about it is it’s just so darn hard to study this, you know, really the best way to do it is to have some sort of airborne platform that can let you go in and around these clouds to get measurements, to try to shed light on that process.
Narrator: “What is the lifetime of a cloud?” That sounds more like a line of poetry than a scientific mission. But it’s an important aspect for the airplane flights of the ACTIVATE mission, because they need time to thoroughly investigate something that’s inherently hazy, ever-changing, and transitory.
[26:02] Armin Sorooshian:When we’re trying to study clouds, I’ve been in situations where we go for a month-long campaign and, unusually, there’s just less clouds that month as compared to other years. Even during a single flight, when we go out there with a single airplane, the way to study these interactions is we typically need to fly below the cloud, in the cloud, above the cloud, and this process takes a while. And by the time you maybe reached the top of the cloud deck, it might not be so representative of what’s going on below the cloud deck anymore.
It’s just hard to do these campaigns. And even if everything works with the airplane and the instruments, you still lack statistics. That’s really a big issue. You know, on a given day, the meteorology can be very different. So you can’t compare the last flight to the current flight. You have to do so many flights where you have enough of the same weather conditions to then compare your data.
The direction we’re trying to head in with this ACTIVATE campaign is to make a conscious effort to build more statistics than normal. We’re doing a lot of repeated types of flights over the same region over the course of three years.
(sound FX: prop plane)
[27:16] So our plan was to have 150 joint flights. And so when I say joint flights, that means instead of doing a single aircraft mission, we’re using two planes. And these two planes fly in a very systematic and synchronized way each flight, where one is low, where most of the clouds are residing that we’re studying, and where most of the pollution sources are. But then there’s a higher plane hovering up at about eight to 10 kilometers, and what it’s doing is it’s using remote sensors to look down at the clouds and aerosols that the bottom plane is flying in.
So the combination of these two provides all the information you need at once, rather than have just one of those planes trying to do everything by itself, which can take a whole lot of time. And our flights are planned for two separate two-month periods each year. Those are in February and March, and then May and June. And the idea there is that if we do flights in different seasons, we’re capturing different weather conditions in different aerosol conditions.
[28:31] Narrator:The ACTIVATE flights take place over the Atlantic, and they fly as low as they safely can near the surface of the ocean, and also high up above the clouds.
Armin Sorooshian:It’s basically characterizing a vertical column that the cloud is evolving in, and we need to understand the whole column from as low as we can get below the cloud to somewhere above the cloud top.
We have a whole range of different ways to do our measurements. We have what are called cloud water probes. These are probes that can stick out from maybe the top or the side of the fuselage, and when we’re in a cloud, cloud water sort of trickles in to these probes, and then we collect them in little vials inside the plane. Sometimes we have remote sensors on these aircraft and those include things like radars, radiometers, LIDARs. And we have what are called inlets, sophisticated tubes that can bring in air from outside of the plane into the inside, and then that air is split up between various instruments. Sometimes we even collect samples on filters and then we take those filters to a lab after the flight to do more advanced chemistry to see what was on the particles that impacted on the filters.
[29:47] Narrator:They also release “dropsondes,” tube-shaped instruments with parachutes that relay important aspects of the atmosphere, like temperature and winds, as they fall down through the cloud to the ocean.
The northwestern Atlantic Ocean region that the ACTIVATE mission focuses on provides a range of different clouds, weather conditions, and aerosols to study. Armin has also worked on many other missions to study clouds and aerosols, usually flying over the ocean.
[30:16] Armin Sorooshian:When you’re close to the ocean, you have sea salt, and sea salt is extremely hygroscopic. That’s a fancy word that means they love to take up water if there’s humidity around. Sea salt is big to begin with, but it’s largely comprised of sodium chloride. Those constituents are very water soluble. They love water. So they take up water better than most other species that are in particles globally.
I didn’t really appreciate it until I started getting into aerosol research of the added influence of shipping. There’s a lot of extensive ship traffic that takes place between places, like Long Beach and Oakland, and those ships, they emit lots of particles in the air, and they’re comprised mostly of sulfate, which again is another very hygroscopic constituent that loves to take up water.
(music)
[31:12] Ship tracks are a very interesting research topic because really to study these aerosol cloud interactions, it’s good to be in a clean area to contrast with a very polluted area. So ships naturally provide that, because off the California coast, for instance, we could be flying in a cloud where you might have 50 droplets per cubic centimeter. And then all of a sudden you’re in an area perturbed by a ship where you can have over a thousand or 500 droplets per cubic centimeter. So that’s a pretty big shift in concentration and that’s perfect for the work we’re doing.
If you have a hundred, a thousand times more droplets in a cloud, they’re going to be much more abundant, but way smaller. And therefore there’s a whole lot more surface area to reflect sunlight. And that’s why can see ship tracks some days in satellite imagery. Go do a Google search on ship tracks and just look at the images; they’re quite remarkable. They’re little lines that exist in these cloud decks close to the ocean, but they’re very bright lines. They’re much brighter than the clouds around them. And they are in very thin straight lines. And, sometimes they curve, but then again, just like jet contrails, you don’t see ship tracks every day.
[32:29] I always look up at the sky, you know, many days you can see those crisscrossing white lines, and then some days you don’t see them at all. It’s not like the jets are not there every day. And then some days you can see them being formed right behind a jet and they don’t last very long. Maybe it’s just a few seconds or a few minutes, and then some days they just hang around. A lot of that is driven just by the environmental conditions that these jets are flying in. So that’s an interesting thing to study. That’s kind of at the heart of these aerosol cloud interactions. Why, for instance, do you see them some days and not other days? Sometimes in fact, when you have ships, they help deplete clouds above them. That again points to the complexity of these interactions.
[33:12] But when we follow these ships, it’s really exciting because there’s a lot of real-time decision-making that has to be done by a flight scientist, which has often been me on these flights. We use a website, it’s called Marine-Traffic-Dot-Org, where in the early morning, before a flight, when we’re planning, we can see where all the major ships are off the coast and where they’ll be during the time of our flights. So we usually select one or two that we think are large enough to create a good signal for us.
Then we go find the ship and it’s usually spot on. It’s at the exact time that the website said it would be at a certain location. And then it’s a matter of zig-zagging where you go behind the ship and turn around and we’re working our way downwind of the ship, but we just keep zig-zagging, and I look at real time data to tell me when we’re in and out of the plume. So we zig-zag and trace that plume as good as we can. And then we might do the same thing at a higher altitude. That’s really some of the most engaging, exciting flying I’ve ever done, doing these ship-chasing flights.
(sound FX: ocean waves)
[34:17] When I look outside the window, just seeing how close we are to the ocean is quite remarkable. We reach sort of the minimum altitude that is safe, but still that’s quite low. And we get close to these ships. We can sometimes see dolphins, even whales. On windy days, you see all the waves breaking.
And these pilots are talented and really into it too. I’ve been impressed throughout my career with these pilots who actually get engaged in science because if they do that, they’re obviously less bored, but they can do the flight maneuvering better if they know what we want.
Narrator:The excitement of flying actually brought Armin to his field of study. He’s been fascinated by planes since he was young, when he spent evenings outside LAX airport during family visits to Los Angeles.
(sound FX: jet taking off)
[35:15] Armin Sorooshian:I would go with my dad and brother, we would just park south of LAX where a lot of plane lovers go and watch planes take off and land, and have a little radio listening to the air traffic control. So I was really big into that. And that’s one of the reasons I got into the work I’m doing now. When I was interested in graduate school, I saw that there’s this research group at Caltech that was doing a lot of airborne research. And I didn’t at the time really fully understand exactly what they were researching, but just knowing that field work can be done with airplanes really hooked me.
Narrator:Despite his love of airplanes Armin never wanted to be a pilot – instead he always wanted to be a scientist, like his parents. They’d come from Iran to work in the United States – his father is a hydrologist who studies rainfall, and his mother is a chemical engineer.
[36:05] Armin Sorooshian:My brother and I are both chemical engineering PhDs, so we followed her path. My dad was a different type of engineer.
One of the interesting things about my family, which is kind of rare, is that we’re part of a religion that’s called Zoroastrianism. It’s arguably the first monotheistic religion that ever existed, and it was part of ancient Persia. So we’re part of that, and it’s an actually really interesting religion that with a big emphasis on Nature. There’s a lot of emphasis on Earth in the form of wind, water, fire, and air. And it’s quite interesting that my dad pursued hydrology, which is the water part of it. And my research is a lot about atmospheric chemistry, so there’s a lot of wind related to that because of dust emissions. There’s fire because biomass burning is a tremendous source of pollution. And air, you know, I study air. So between the two of us, we study those four pillars of Nature that’s such a big part of the religion.
[37:07] I should also say I’m not terribly religious myself, I just know bits and pieces from growing up. I couldn’t tell you too many details about what’s said about those four, other than they are precious parts of our planet and that we respect them greatly. And that they’re things that are meant to be clean and pure and we rely on them for life.
And the other thing about the religion is we go by three principles, and they’re good words, good thoughts and good deeds. And the idea is if you follow these, you will end up going to a good place when you pass away. Probably our most famous Zoroastrian, of course, is the late great Freddy Mercury.
Queen song: We Are the Champions
We are the champions
We are the champions
No time for losers
‘Cause we are the champions
Of the world
[38:12] Narrator:Aerosol particles might be some of the tiniest things around, but they can make powerful impacts on our world.
Armin Sorooshian:A lot of our research has focused on things like weather and climate, but public health is a huge deal. The World Health Organization said that the leading cause of death globally due to environmental threats is aerosols. And interestingly enough, most of the deaths are due to indoor air pollution, not even outdoor air pollution. That’s due to some parts of the world where there’s indoor cooking and poor ventilation, things like that. That’s a huge problem.
Just with the current pandemic, I think there’s a growing appreciation of what aerosols are now. COVID is transferred very effectively between humans just by talking, you know, you talk, and these aerosols coming out of your mouth, they’re airborne. They’re particles or little droplets, and they can just hang in the room and someone can inhale it.
[39:09] And even things like dust can have a tremendous impact on health. Between Tucson and Phoenix, one of the scariest areas to drive is on Interstate 10, just north of a little place called Picacho Peak. I’ve driven by this thing hundreds of times, but on a couple of occasions, I was terrified for my life because there was just a small little dust storm, small in that a model probably couldn’t have predicted, and it was not very long, but it reduces visibility down to nearly zero. You can’t see anything in front of you on the Interstate. You know, these small-scale events like on Interstate 10, it’s not that we’re inhaling the dust and dying from that; it’s just traffic accidents. People don’t know what to do. Do they pull over? Do they slow down? Do they speed up? So the impacts on visibility can be quite dramatic, leading to fatalities.
So that’s an example of these small-scale events, with things like dust, that climate models or regional-scale models can’t predict well, because they happen at such fine resolution. It could be just a little wind event that stirred that up.
[40:17] Narrator:Tiny aerosols also play an outsized role in climate change. We’re all now aware that gases like carbon dioxide are causing our world to grow warmer, but Armin says aerosols complicate that picture.
Armin Sorooshian:So when we talk about pollution, it’s important to separate gases from the particles. So the warming to be concerned about is driven by greenhouse gases. These particles are very different in that they’re much bigger than the gases. And so when Sun shines down, that solar radiation, it’s actually reflected by the particles back up to space. The gases don’t reflect sunlight back up, instead gases trap that radiation and send it back down, increasing temperatures.
[41:03] So we have some idea of how the cooling by these aerosols can compete with the warming by the gases. Since particles reflect the sunlight, they have a net cooling effect. There’s some particles that can lead to warming, like black carbon, the black stuff you see coming out of some sources like tailpipes. That actually can absorb and warm. But most other types of particles that have scattering properties, they could reflect and cool.
(music)
Greenhouse gases actually have a pretty high level of confidence in terms of the warming they cause. But aerosols, their ability to cool is a little bit of a larger uncertainty. We don’t fully understand the magnitude of their cooling. The very largest uncertainty has to do with how aerosols can indirectly cool the planet because of cloud formation. I think that the understanding has improved over time, but there’s still a lot more to be learned.
(music)
[42:05] You know, there’s reports of these ancient philosophers who spoke about clouds. And the philosopher in mind is Rene Descartes, who noted that the most complex thing in Nature is clouds. And that if one can actually explain the nature of clouds, then man can explain almost anything in Nature.
(music)
Narrator: If you like this podcast, please subscribe, rate us on your podcast platform, and share us on social media. We’re “On a Mission,” a podcast of NASA’s Jet Propulsion Laboratory.
[run time = 42:48]