A Flexible, Personalized, On-Demand Astropharmacy
Lynn Rothschild
NASA Ames Research Center
Disease is an inherent part of being alive, and thus disease prevention, diagnosis and treatment is critical to human space missions. Pharmaceuticals are used to diagnose, treat, cure, or prevent disease, but suffer from lack of stability on Earth and even more so in the space environment. As NASA embarks on a new era in space exploration beyond Low Earth Orbit, the need to provide effective pharmaceuticals in space must be addressed. What if small quantities of pharmaceuticals could be made in space, on site, on demand? One class of drugs poses the greatest challenge: small protein (peptide) drugs. These drugs, even with refrigeration, have the shortest shelf-life (months), and therefore require a “production-on-demand” solution for long duration missions that may last years. The protein drugs—approximately 1/3 of all new drugs that come to market today—include some of the most important spaceflight countermeasures, such as filgrastim, a growth factor that can restore the bone marrow after radiation damage, and teriparatide, a peptide drug for preventing bone demineralization.
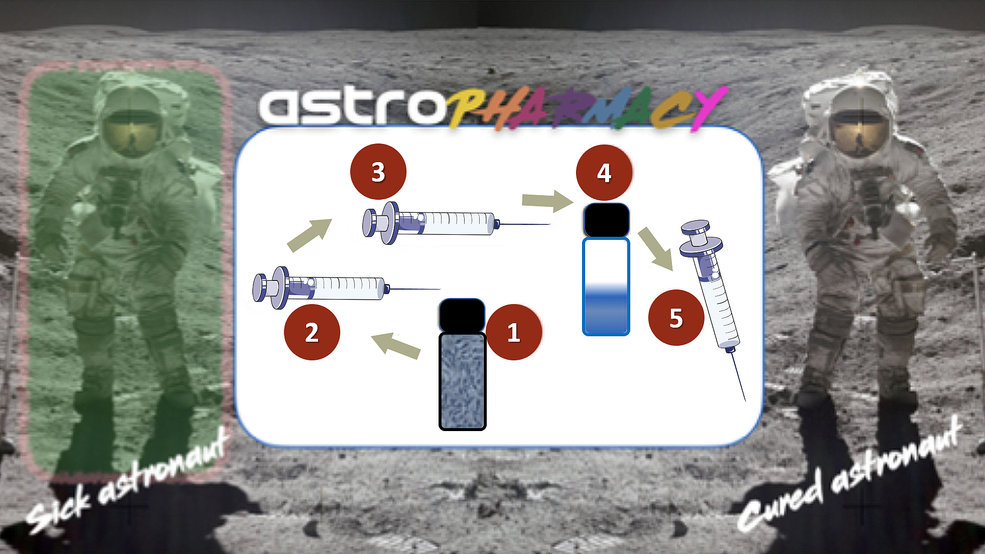
In Phase I we introduced our production-on-demand solution, which we call “Astropharmacy”, a platform technology that involves the synthesis of drugs by a space-hardy spore-forming bacterium, Bacillus subtilis, together with a novel purification system that uses histidine tags for affinity purification. We engineered B. subtilis to produce the non-glycosylated drugs filgrastim and teriparatide, which we purified with a lightweight, small volume system adapted from standard laboratory protocols and enabled by judicious genetic engineering prior to launch.
Our Phase I effort was successful, but the key unknown remains: Can we produce drugs in our system of sufficient quantity and purity to be useful to astronauts? This Phase II addresses this unknown in several ways through these objectives:
- Evolution: We will further our analysis of the use of this system by conducting a paper study of other drugs in the astronaut medication list to identify those that could be produced with our system, including drugs with post-translational modifications that may require a cell free or a yeast production platform.
- Technical: Further develop engineering techniques for drug production and secretion, including producing 3 drugs in addition to those produced in Phase I. A key finding of Phase I is that optimal production can prove toxic to the production cell, so a cell free platform may be desirable. Assays for purity will be developed for product validation testing using industry standard bio-assays to demonstrate that the Astropharmacy produces drugs with the expected biological activity. Stability during a long-duration mission is the key driver, so testing of the cellular system and cell free systems will take place over time and under simulated extraterrestrial conditions. We will prototype a low mass microfluidics-based production/purification system and use it for drug production. We will create a technology roadmap to implementation beyond Phase II.
- Representative mission: We will analyze deployment in the context of Mars mission with particular focus on the occurrence of a solar particle event. We have a time-critical opportunity to test the protocol for Astropharmacy during a lunar analog mission, Asclepios III, to take place Summer 2023. Two of our students have been selected as mission “astronauts”.
- Impact: We have begun discussions with DoD (e.g., Space Force, AF) and will continue for Phase II for this customer, as well as industry. Through student involvement we will build on our Phase I workforce development, as well as continue with press attention to inspire the public.
If successful, our Astropharmacy will be a critical advance that will support medical operations on long duration deep space missions, as well as spin-off applications on Earth.